|
 |
| Organoid > Volume 3; 2023 > Article |
|
Abstract
NOTES
Funding
This work was supported by the National Research Foundation of Korea (NRF; Grant No. NRF-2021R1A3B1077481). This research was financially supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for Advancement of Technology (KIAT) through the International Cooperative R&D program (P0011266).
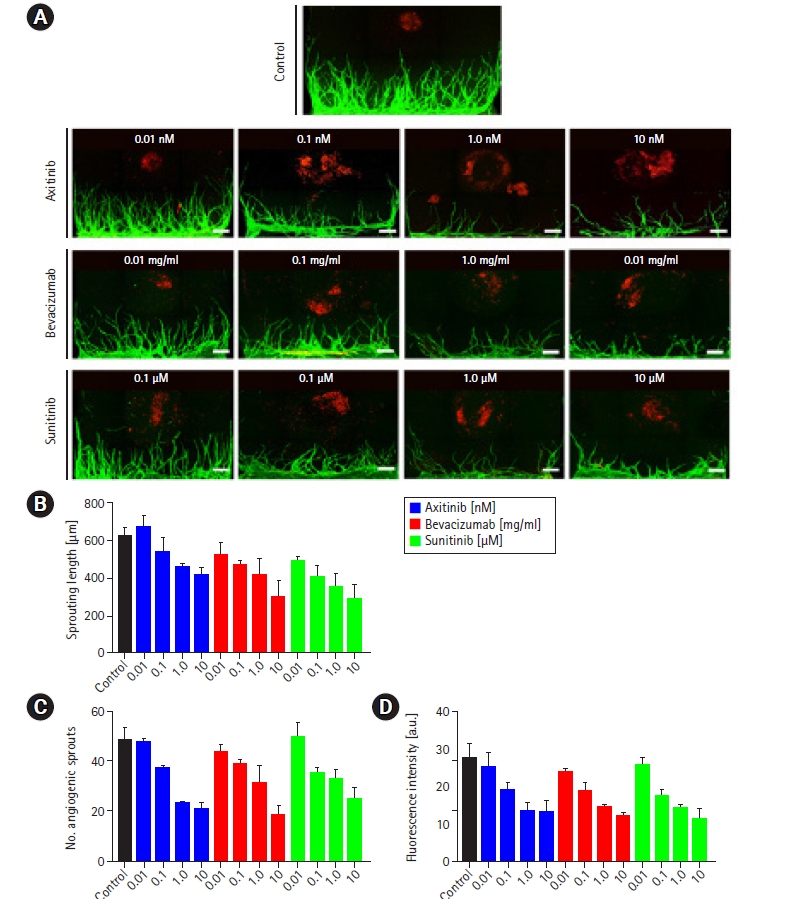
Fig.┬Ā1.
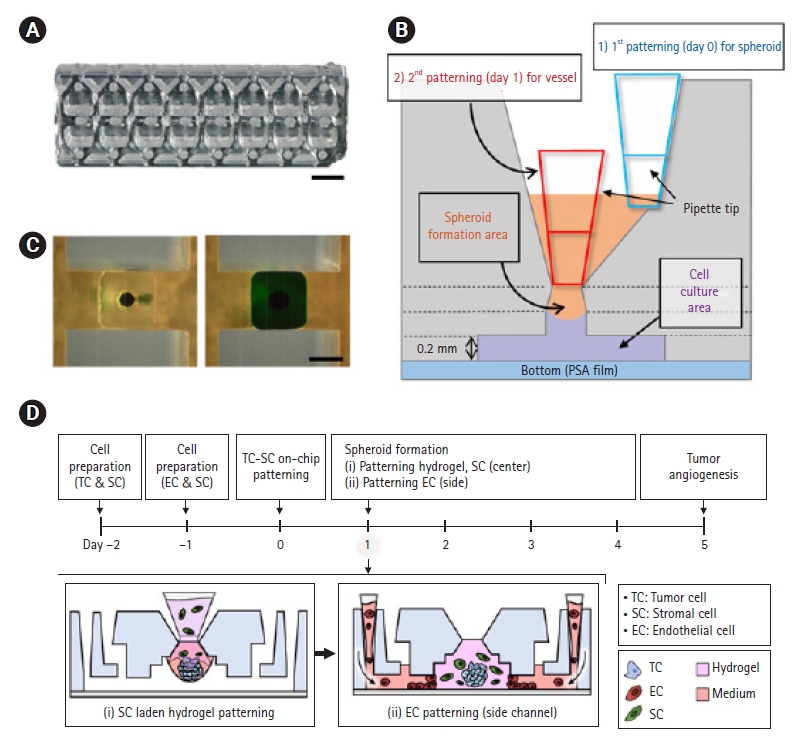
Fig.┬Ā2.
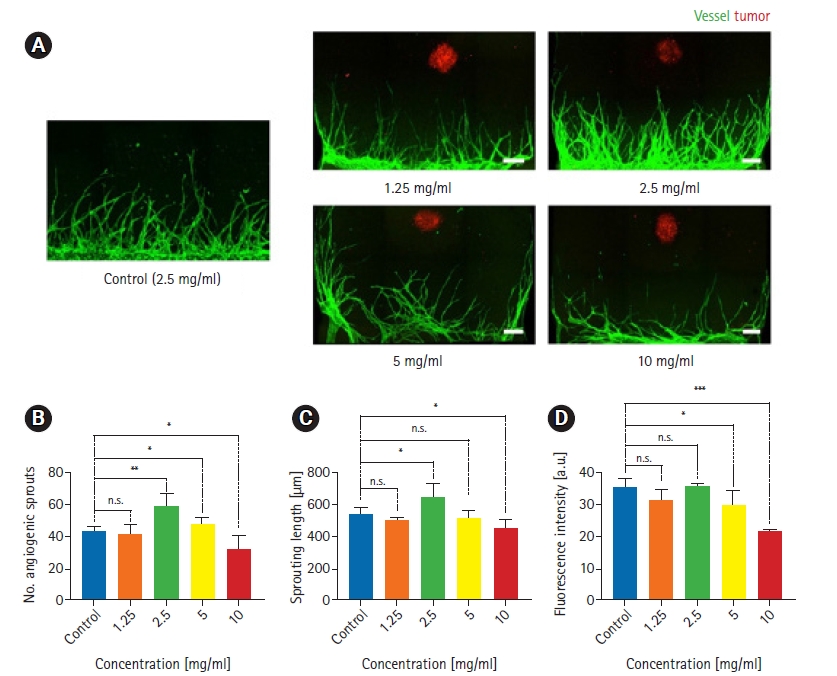
Fig.┬Ā3.
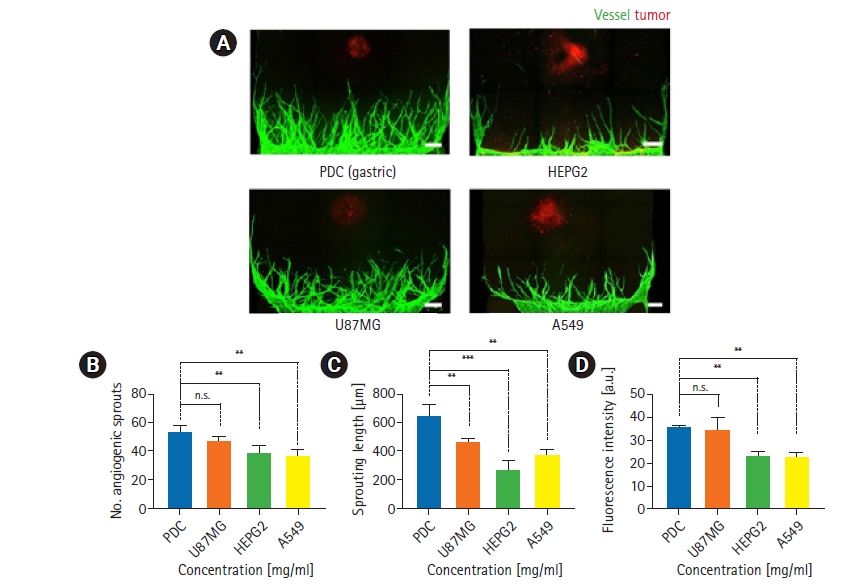
Fig.┬Ā4.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,409 View
- 53 Download
- ORCID iDs
-
Seonghyuk Park

https://orcid.org/0009-0004-8946-1993Youngtaek Kim

https://orcid.org/0000-0002-4654-5925Jihoon Ko

https://orcid.org/0000-0002-6555-3728Jeeyun Lee

https://orcid.org/0000-0002-4911-6165Young-Kwon Hong

https://orcid.org/0000-0001-8245-875XNoo Li Jeon

https://orcid.org/0000-0003-0490-3592 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print